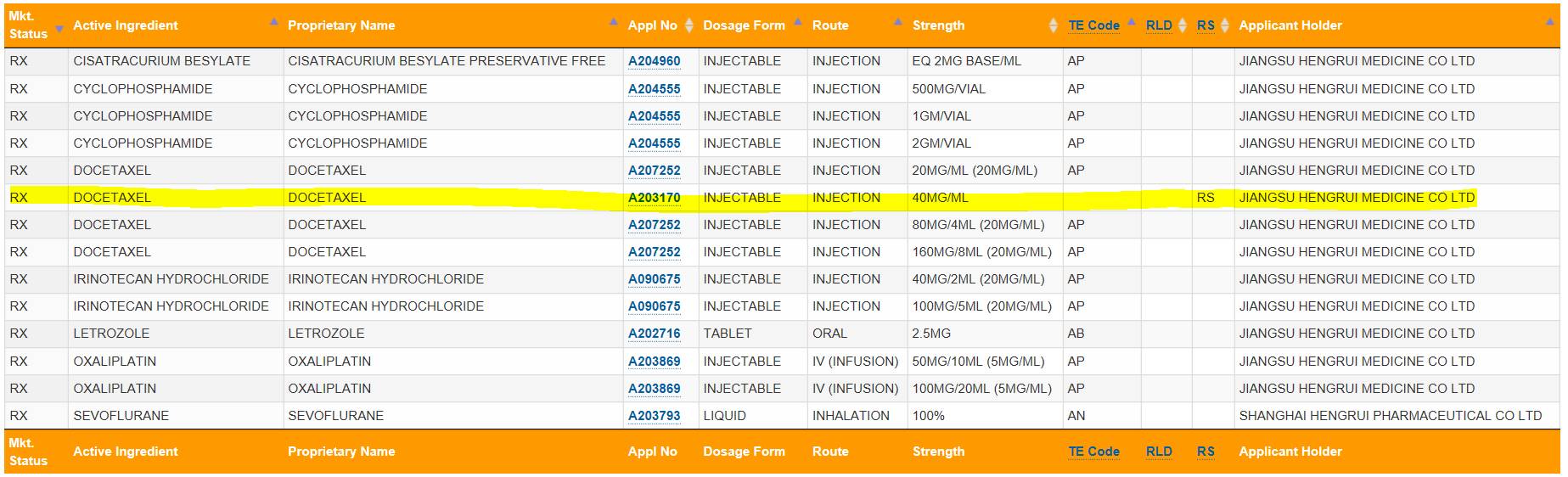
本月9号,美国FDA批准了恒瑞医药的多西他赛注射液上市。其中40mg/ml被FDA指定为参比制剂(RS),ANDA号为 A203170
Active Ingredient: DOCETAXEL
Proprietary Name: DOCETAXEL
Dosage Form; Route of Administration: INJECTABLE; INJECTION
Strength: 40MG/ML
Reference Listed Drug: No
Reference Standard: Yes
TE Code:
Application Number: A203170
Product Number: 001
Approval Date: Feb 15, 2017
Applicant Holder Full Name: JIANGSU HENGRUI MEDICINE CO LTD
Marketing Status: Prescription
Patent and Exclusivity Information